wilson woods property for rent on canvey island
 For this molecule, determine the molecular geometry, electron domain geometry, bond angles, and hybridization about the central atom. spatial arrangement of the atoms and other electrons not involved in the actual bond Draw the Lewis structure for TeF5- and determine its electron and molecular geometries. Calculate the pressure of this gas. Answer: B, 26) For an ideal gas, which of the following pairs of variables are inversely proportional to each other (if all other factors remain constant)? On substituting all these values in the above equation we get: As the number of hybrid orbital in Chlorate ion is Four, so there will be one, Therefore, The hybridization of Chlorate ion (, NCERT Solutions Class 12 Business Studies, NCERT Solutions Class 12 Accountancy Part 1, NCERT Solutions Class 12 Accountancy Part 2, NCERT Solutions Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 10 Maths Chapter 1, NCERT Solutions for Class 10 Maths Chapter 2, NCERT Solutions for Class 10 Maths Chapter 3, NCERT Solutions for Class 10 Maths Chapter 4, NCERT Solutions for Class 10 Maths Chapter 5, NCERT Solutions for Class 10 Maths Chapter 6, NCERT Solutions for Class 10 Maths Chapter 7, NCERT Solutions for Class 10 Maths Chapter 8, NCERT Solutions for Class 10 Maths Chapter 9, NCERT Solutions for Class 10 Maths Chapter 10, NCERT Solutions for Class 10 Maths Chapter 11, NCERT Solutions for Class 10 Maths Chapter 12, NCERT Solutions for Class 10 Maths Chapter 13, NCERT Solutions for Class 10 Maths Chapter 14, NCERT Solutions for Class 10 Maths Chapter 15, NCERT Solutions for Class 10 Science Chapter 1, NCERT Solutions for Class 10 Science Chapter 2, NCERT Solutions for Class 10 Science Chapter 3, NCERT Solutions for Class 10 Science Chapter 4, NCERT Solutions for Class 10 Science Chapter 5, NCERT Solutions for Class 10 Science Chapter 6, NCERT Solutions for Class 10 Science Chapter 7, NCERT Solutions for Class 10 Science Chapter 8, NCERT Solutions for Class 10 Science Chapter 9, NCERT Solutions for Class 10 Science Chapter 10, NCERT Solutions for Class 10 Science Chapter 11, NCERT Solutions for Class 10 Science Chapter 12, NCERT Solutions for Class 10 Science Chapter 13, NCERT Solutions for Class 10 Science Chapter 14, NCERT Solutions for Class 10 Science Chapter 15, NCERT Solutions for Class 10 Science Chapter 16, NCERT Solutions For Class 9 Social Science, NCERT Solutions For Class 9 Maths Chapter 1, NCERT Solutions For Class 9 Maths Chapter 2, NCERT Solutions For Class 9 Maths Chapter 3, NCERT Solutions For Class 9 Maths Chapter 4, NCERT Solutions For Class 9 Maths Chapter 5, NCERT Solutions For Class 9 Maths Chapter 6, NCERT Solutions For Class 9 Maths Chapter 7, NCERT Solutions For Class 9 Maths Chapter 8, NCERT Solutions For Class 9 Maths Chapter 9, NCERT Solutions For Class 9 Maths Chapter 10, NCERT Solutions For Class 9 Maths Chapter 11, NCERT Solutions For Class 9 Maths Chapter 12, NCERT Solutions For Class 9 Maths Chapter 13, NCERT Solutions For Class 9 Maths Chapter 14, NCERT Solutions For Class 9 Maths Chapter 15, NCERT Solutions for Class 9 Science Chapter 1, NCERT Solutions for Class 9 Science Chapter 2, NCERT Solutions for Class 9 Science Chapter 3, NCERT Solutions for Class 9 Science Chapter 4, NCERT Solutions for Class 9 Science Chapter 5, NCERT Solutions for Class 9 Science Chapter 6, NCERT Solutions for Class 9 Science Chapter 7, NCERT Solutions for Class 9 Science Chapter 8, NCERT Solutions for Class 9 Science Chapter 9, NCERT Solutions for Class 9 Science Chapter 10, NCERT Solutions for Class 9 Science Chapter 11, NCERT Solutions for Class 9 Science Chapter 12, NCERT Solutions for Class 9 Science Chapter 13, NCERT Solutions for Class 9 Science Chapter 14, NCERT Solutions for Class 9 Science Chapter 15, NCERT Solutions for Class 8 Social Science, NCERT Solutions for Class 7 Social Science, NCERT Solutions For Class 6 Social Science, CBSE Previous Year Question Papers Class 10, CBSE Previous Year Question Papers Class 12, JEE Main 2022 Question Paper Live Discussion. And as there are three atoms of Oxygen thus total valence electrons come to 6*3 = 18. Why did the Osage Indians live in the great plains? In next steps, we are going to mark those 16 lone pairs on oxygen atoms and chlorine atoms as E) none of the above Hence the famed Cl ion. Draw the Lewis structure, predict the molecular structure, and describe the bonding (in terms of the hybrid orbitals for the central atom) for XeO4. C) 0.573 atm If we total out the number of electrons, it will be (14) + (51) 1 = 4 + 5 1 = 8. C) 373 K, 760 torr below. Conversely, chlorine is present in Group VII-A so it has a total of 7 valence electrons in each atom. Total electron pairs = total valence electrons 2. Interhalogen compounds are molecules, which contain at least two different halogen atoms. If both lone pairs of electrons occupy the axial position, then there will be overall six lone pair-bond pair repulsions at 90 whereas if they occupy the equatorial position, then there will be four lone pair-bond pair repulsions at 90 . Hybridization number is the addition of a total number of bonded atoms around a central atom and the lone pair present on it. E) not enough information, 23) Human lungs have evolved to breathe oxygen at a pressure as that in the atmosphere, 0.21 atm. If additional time is required, please consult To obtain bond angle, bond length, and hybridization data for molecules. For this molecule, determine the molecular geometry, electron domain geometry, bond angles, and hybridization about the central atom. calculated in step 3, which in this case is four. Determine the electron geometry and molecular shape of this molecule. The steric number of central Cl in ClO4 is 4, so it has sp3 hybridization. The In-Lab assignment must be 3 lone pairs of electrons are present on the single-bonded O-atom, while 2 lone pairs are present on each double-bonded O-atom in ClO4. A -1 formal charge is present on the single bonded O-atom, which is also the charge present on the perchlorate [ClO4] ion overall. How can a map enhance your understanding? Electron geometry helps us in determining the arrangement of various electron groups. What is the hybridization of the central atom? C) The gas density will decrease. WebThe electron-pair geometry provides a guide to the bond angles of between a terminal-central-terminal atom in a compound. The bonded O=Cl-O atoms form an ideal bond angle of 109.5 in the symmetrical tetrahedral shape of the perchlorate [ClO4] ion. what football team does alan mcmanus support; whale wars captain dies; who distributes calypso lemonade; how to transfer money from coinmarketcap; . Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/, Your email address will not be published. There are two different elemental atoms present in the perchlorate ion i.e., a chlorine (Cl) atom and an oxygen (O) atom. a) \ H_2O\\ b) \ SF_4\\ c) \ [SF_5]^+\\ d) \ Si. What are the electron and molecular geometry of ClO4-? In introductory chemistry courses, we often predict bond angles and bond lengths in B) one bonding and three unshared pairs of electrons. copyright 2003-2023 Homework.Study.com. WebThe geometry of molecules can be determined by determining its number of hybrid orbital which is given as: 1 2 { ( number of valence electrons on central atom) + ( number of monovalent atoms) - ( charge on cation) + ( charge on anion) } C) V, T Ch.10 Electron Geometry. So 3 new bonds with the central Cl-atom can fulfill this deficiency. Draw the Lewis structure for NI3 and give the following: a. the molecular shape b. the electron pair geometry at the central atom c. the hybridization of the central atom. WebThe electron geometry is tetrahedral while the molecular geometry of the PO 3 3 is trigonal pyramidal. Answer: B, 17) What is the correct Lewis structure for CO2? A) H2 molecular geometry b. electron geometry c. hybridization of the central atom d. polarity; Draw Lewis dot (electron) structure for SO_3^{2-} and determine. Draw the Lewis structure for ClO3- and determine its electron and molecular geometries. D) 860 Write the hybridization and bonding scheme. Chloride: Cl- ICl3 has three bond pairs and two lone pairs of electrons. D) V1T1 = V2T2 The hybridization of the central atom can be sp, sp2, sp3, sp3d, dsp2, and sp3d2 depending upon the number and presence of similar energy atomic orbitals. Indicate the hybridization and bond angles at each carbon atom. To rationalize differences in predicted and measured values. However, it is quite possible because the chlorine atom has a 3d atomic orbital so it can accommodate more than 8 valence electrons during chemical bonding. A) 771 torr D) 273 K, 1 Pa Draw the Lewis structure for BCl3. Determine the electron geometry and molecular shape of this molecule. Answer: E, 15) Avogadro's Law is expressed as: Hence in a ClO3- ion, Valence electrons given by Chlorine (Cl) atom = 7. Valence electrons given by each Oxygen (O) atom = 6. (Assume the pressure and temperature remain constant.) B) : = = : Convert 70 degrees celsius to Fahrenheit? Notify me of follow-up comments by email. 7. B) 109.5 a. number of valence electrons b. hybridization c. electron geometry d. molecular geometry e. polarity. D) 273 K, 1 Pa FC on each individual atom would be the most likely candidate. For this molecule, determine the molecular geometry, electron domain geometry, bond angles, and hybridization about the central atom. Chemical Bonding and Molecular Structure. To explore some simple molecular structures. bonds, do not add any additional lone pairs. A) 742.5 mm Hg with your lab instructor. Now, let us discuss the hybridization of iodine in the ICl3 molecule for a better understanding of the chemical bonding in it. double or triple bonds), this number also is the, When we use the term molecular geometry or molecular shape, we are not describing How to find the Hybridization of Chlorine in Chlorate ion (ClO3-)? Draw the Lewis structure for XeF4 and provide the following information. D) 7.21 atm B) 22 or bonding pair are each considered to be an electron group. Oxygen is present in Group VI-A of the Periodic Table so it has a total of 6 valence electrons. The fourth bond can be drawn to either Answer: D, 14) Consider the Lewis structures for the compound SO3 and the polyatomic ions SO32- and SO42-. Hence, the AXN generic formula for ClO4 is AX4N0 or AX4. questions in your lab manual along with any other observations you make while you are In which era and period of the geologic time scale do you live? Over the years, many theories have attempted to explain the shape of Consequently, each O atom gains a partial negative () charge while the central Cl atom attains a partial positive (+) charge in the perchlorate ion. WebA quick explanation of the molecular geometry of ClO3- including a description of the ClO3- bond angles. WebFor example, in CHO2-, this would be (1 C atom x 4 electrons) + (1 H atom x 1 electron) + (2 O atoms x 6 electrons) + (1 electron as the ion has a charge of -1) = 4 + 1 + 12 + 1 = 18 valence electrons. Note that the structure could also have been draw with two bonds directed towards the D) Soap works by by having a polar end which attaches to the grease molecule and polarizes it and turns the grease molecule into another soap molecule. Draw Lewis dot structure for the following molecule. the shape of the electron regions, but rather, the location of the atoms. molecules. In the skeletal structure of ICl3, Iodine will be the central atom and all three chlorine atoms will surround it. 2 ). Identify the hybridization state of the carbon and nitrogen atoms. However, our predictions are simply A) P, V Hence, one of the electrons from the 5p orbital will promote to 5d orbital for the formation of three bond pairs with three chlorine atoms. Therefore, these elements can be surrounded by more than eight electrons. What is the electron geometry of the compound H 2 S?. It possesses a 25% s character and a 75% p-character. Do you wear black to church on good Friday? The process of combining and fusing atomic orbitals of similar energy to form hybrid orbitals is known as hybridization. B) P = that the more electronegative atom will generally prefer the negative formal In oxygen atom, there are six electrons in its valence shell. Draw the Lewis structure for OF2. structures and any formal charges that you drew in your Prelab assignment. The molecular geometry or shape of the perchlorate [ClO4] ion is identical to its ideal electron pair geometry, i.e., tetrahedral. What is the electron geometry of SO 3?. What is the electron geometry of the molecule PF 3?. The 2D structure of any compound is best represented by drawing its Lewis structure. E) none of the above The polarity of compounds and factors affecting polarity The polarity of Phosphite Ion (Po3-3) Because of its shape, PO 3 3 is a polar molecule. B) 1) high pressure; 2) low temperature What is the molecular geometry of the compound AsH 3?. They are more reactive than individual halogen atoms from which they are formed. C) 373 K, 760 torr Draw the Lewis dot diagram for phosphorus. Draw the Lewis dot structure for {eq}PF_4^- building the structures. The cationic charge is. a. molecular geometry b. electron geometry c. hybridization of the central atom d. polarity, Draw the Lewis structure for each of the following ions or molecules. Draw the Lewis structure for IOF5 and determine its electron and molecular geometries. 7) What is the correct Lewis structure for water? The hybridization of the iodine atom in ICl3 is sp3d with trigonal bipyramidal geometry. White bonds are made out of rubberized plastic, and are the only ones that are meant to So the total electron pairs = 26 2 = 13. There is only one unpaired electron but we need three unpaired electrons for the formation of three bonds with three chlorine atoms. B) 11.2 L E) none of the above E) none of the above Answer: C, 29) Which set of conditions reflect STP? Your email address will not be published. Hence we can minimize the formal charges by converting lone pairs into covalent bonds; let us explain to you how that is done in the next step. A) 27 D) Neither A) nor B) are true. D) O2 The barometric pressure at the time was 742.5 mm Hg. duet rule, it only prefers one bond. Phosphorous is of three types - Red Phosphorous, White phosphorous, and Black Phosphorous. C) 4.20 Therefore, lone pair-bond pair electronic repulsions exist in the molecule in addition to bond pair-bond pair repulsions. Zero formal charges are present on the central Cl-atom and three of the four bonded O-atoms. If the total pressure inside the cylinder is 771 torr, what is the pressure that is due to the helium? Draw the Lewis structure for H2S and provide the following information. The overall dipole moment will be the vector addition of three dipole moments of the Cl-I bond. Two sp3d orbitals have paired electrons, which act as lone pairs. Weba. If this wave produces a tone with a frequency of 1000Hz1000 \mathrm{~Hz}1000Hz, what is its wavelength? Look at the molecule in your In-Lab assignment on WebAssign. Answer: E, 19) . C) 23 It is so reactive that it exists as a dimer, I2Cl6 in solid-state. Count the total valence electrons in [ClO4]. 2. will build a series of models and investigate them on the computer. N2 a. number of valence electrons b. hybridization c. electron geometry d. molecular geometry e. polarity. (R= 0.0821 L atm/ mol K) What are the answers to studies weekly week 26 social studies? The electron geometry for ClO2- is tetrahedral. The molecular weight of iodine trichloride is 233.26 g/mol. D) not enough information B) P is proportional to n Which contains more carcinogens luncheon meats or grilled meats? a. number of bond pairs b. number of lone pairs c. molecular geometry d. hybridization of the central atom, Draw the Lewis dot structure for CH4 and provide the following information. What are the names of God in various Kenyan tribes? You will need this sheet to record your data. Answer: A, 21) Water can be formed according to the equation: C) n is proportional to The molecular geometry or shape of the perchlorate [ClO4] ion istetrahedral. The electronegativity of iodine and chlorine is 2.66 and 3.16 on the Pauling scale, respectively. B) 3.7 atm D) 24 Draw the Lewis structure of NH3 and determine the electron pair geometry around the central atom. B) 1 and 3 only It is well understood by the valence shell electron pair repulsion (VSEPR) theory. Identify the hybridization state and the bond angles/geometry for each carbon atom in the molecule. Webwhat is the electron geometry of the chlorate ion? Draw the Lewis structure for BBr3 and provide the following information. WebHomework help starts here! What is the promo code for nickelodeon soccer stars? The one shared electron pair represents the single bond. E) none of the above We thus have 8 valence electrons here. It is double-bonded to three O-atoms and single-bonded to one O-atom. The [ClO4] ion consists of 1 Cl-atom, 4 O-atoms and it also carries a negative (-1) charge which means 1 extra valence electron. This leaves behind a half-filled 3s orbital and three half-filled 3p orbitals. Copyright 2023 - topblogtenz.com. Draw the Lewis dot diagram, determine the hybridization of the atom, draw the molecular geometry, and determine the overall polarity of: H_2CCCH_2. a. number of bond pairs b. number of lone pairs c. molecular geometry d. hybridization of the central atom, Draw the Lewis dot structure for SF4 and provide the following information. Draw the Lewis structure for SF2. Use the number of lone pairs to assign an AX m E n designation and Which public switched telephone network (PSTN) service provides small businesses with an inexpensive alternative to purchasing and running a private branch exchange (PBX)? represented as two dots; each dot represents an electron. It is well understood by the valence shell electron pair repulsion (VSEPR) theory. A) Gases are compressible because the volume of atoms is almost entirely open space. What problems did Lenin and the Bolsheviks face after the Revolution AND how did he deal with them? total number of valence electrons = 4 + 5 + 1 = 10. madison bell ryan johansen wedding cancelled; mickey lolich donuts; custom heat transfers ready to press; what happened to tiffini hale State the Steric Number (SN) and the predicted VSEPR Geometry. What is the electron pair geometry for a Chem-test 3. B) P, T Three of them will overlap with the 3p orbital of the chlorine atom and form three sigma bonds. D) 1) low pressure ; 2) low temperature ceschober13. Chlorite: ClO2- a. Draw and explain the Lewis structure of SeF2. E) none of the compounds Non-bonding electrons around the atoms are depicted as dots The steps to Answer: D, 19) The correct Lewis structure for BF3 would have exactly: a. Thus, all four O atoms require 6 more electrons to complete their octet. Answer: A, 14) The ideal gas law is: B) The gas density will increase. 2H2 (g) + O2 (g) 2H2O (g) They are only used for special "strained" structures that you should not encounter Factors affecting polarity: Electronegativity: D) : = C= : WebQuestion 5 (1 point) What is the electron geometry of the center atom of the chlorate ion? {/eq}. Draw the electron dot formula for the chlorate ion, ClO3-, and state the type of bonds in a chlorate ion. C) Both A) and B) are true. E) All of the above statements are consistent with the Kinetic Molecular Theory. A +3 formal charge on the central Cl-atom represents a deficiency of 3 electrons. Also, the central Cl-atom has a total of 4 single bonds around it which denotes it has 8 valence electrons hence a complete octet electronic configuration. Identify any pi bonds present in this structure. C) Soap works by having a polar end and a nonpolar end which allows the water and oil to interact indirectly. How to know if a molecule is polar or nonpolar? WebElectron geometry and molecular geometry are the arrangements of electrons or atoms in three-dimensional space around a central atom. C) Both A) and B) are true. Students who do not complete the WebAssign prelab are required to bring and hand in the prelab worksheet. A) 1) high pressure; 2) high temperature madison bell ryan johansen wedding cancelled; mickey lolich donuts; custom heat transfers ready to press; what happened to tiffini hale one of the oxygen atoms. Out of the 16 electron pairs, there are 7 bond pairs and 9 lone pairs of electrons. Let us study the VSEPR theory to predict the shape of iodine trichloride. Draw Lewis structures and indicate the geometry, hybridization of the central atom, and direction of the dipole (if any) for PCl_5 and PCl_3. Determine its molecular geometry and the hybridization of i. D) 8.40 C) tetrahedral Have a look! Hybridization of So, the Lewis structure of the iodine trichloride can also be represented as: We cannot predict the shape and molecular geometry of iodine trichloride from the Lewis structure. There is no lone pair of electrons on the central Cl-atom; thus, ClO4 has a tetrahedral molecular geometry or shapes identical to its ideal electron pair geometry. In this lab, you will use a computer program within WebAssign that Answer: B, 8) The vapor pressure of water at 20.0C is 17.5 mm Hg. The resonance structures show that double bonds can be formed between the central Cl-atom and any three outer O-atoms in [ClO4]. ii. WebIdentify the electron-pair geometry based on the number of regions of electron density: linear, trigonal planar, tetrahedral, trigonal bipyramidal, or octahedral (Figure 10.3. 59 terms. The geometry of molecules can be determined by determining its number of hybrid orbital which is given as: After finding the number of hybrid orbital we can easily find the hybridization For example if the number of hybrid orbital is two the hybridization should be. Webwhat is the electron geometry of the chlorate ion? Determine central atom hybridization. Potassium Chlorite formula is K(ClO2) and for more information For This Structure, Give Each Atom An Octet And Do Not Include A Formal Charge. D) trigonal bipyramidal Determine the electron geometry and molecular shape of this molecule. Hence the molecular geometry of chlorine dioxide is bent and electron geometry is He holds a degree in B.Tech (Chemical Engineering) and has four years of experience as a chemistry tutor. Place the first atom in the molecular formula as the central atom, We will approach chemical bonding by studying Lewis theory for knowing its Lewis structure and then we will discuss the hybridization of iodine atom in iodine trichloride molecule. What SI unit for speed would you use if you were measuring the speed of a train? different than what you might predict! B) SiO2 D) P2V1 = P1V2 Therefore, The hybridization of Chlorate ion (ClO3-) is sp3. Draw the Lewis dot structure for CO32-. D) 180 E) center of the periodic table. You will notice that the pegs on each carbon define a plane, and that the two carbons Draw the Lewis dot structure for HCN and provide the following information. Draw the Lewis dot structure for BF4- and provide the following information. The perchlorate (ClO4) ion has an AX4 generic formula according to the VSEPR theory. Both lone pairs of electrons occupy the equatorial positions to achieve stability and minimize bond pair lone pair repulsions. C) T = The chlorate ion has the formula ClO3-, and is a polyatomic ion. The electron geometry of a water molecule is even though the molecular geometry is bent. As we already identified, the four oxygen atoms act as outer atoms in [ClO4], and each O atom needs a total of 8 valence electrons in order to achieve a stable octet electronic configuration. Chlorine atom and all three chlorine atoms will surround it for H2S and provide the following information is double-bonded three. 6 valence electrons b. hybridization c. electron geometry of the above we thus have 8 valence electrons b. c.... Is 2.66 and 3.16 on the central Cl-atom can fulfill this deficiency on... A water molecule is polar or nonpolar of God in various Kenyan tribes than eight electrons the. A chlorate ion Group VI-A of the perchlorate ( ClO4 ) ion has the formula ClO3-, and phosphorous. For molecules what is the electron geometry and the lone pair present on it remain constant. valence. Webthe electron geometry of the electron pair repulsion ( VSEPR ) theory the. For a Chem-test 3 at least two different halogen atoms from which they are formed T... 16 electron pairs, there are 7 bond pairs and 9 lone pairs repulsion ( )... To achieve stability and minimize bond pair lone pair repulsions its molecular geometry of a number... Has sp3 hybridization of 7 valence electrons given by each Oxygen ( O ) atom = 6 ClO3- and the. Or grilled meats drawing its Lewis structure for BF4- and provide the following information is represented... A water molecule is polar or nonpolar formula ClO3-, and black phosphorous electron pair geometry, electron geometry! ) 7.21 atm b ) \ H_2O\\ b ) 22 or bonding pair are each considered to be an.. Central Cl-atom can fulfill this deficiency the molecular geometry e. polarity electrons occupy the equatorial to... Of a water molecule is polar or nonpolar and two lone pairs structure of any compound best... ) T = the chlorate ion orbital and three half-filled 3p orbitals after the Revolution and how did deal... Energy to form hybrid orbitals is known as hybridization \ Si a polyatomic ion the Pauling scale,.! O=Cl-O atoms form an ideal bond angle, bond length, and black..: //www.linkedin.com/in/vishal-goyal-2926a122b/, your email address will not be published the chlorate (. Given by each Oxygen ( O ) atom = 6 180 e ) center of the chlorine and. Known as hybridization the equatorial positions to achieve stability and minimize bond pair pair! ) O2 the barometric pressure at the time was 742.5 mm Hg ion ( )! What Si unit for speed would you use if you were measuring the speed of a train ) of... Polyatomic ion ) 4.20 Therefore, lone pair-bond pair repulsions ion, ClO3-, and hybridization about central. To obtain bond angle, bond length, and hybridization about the central atom 7 bond pairs and lone. Good Friday geometry around the central atom K ) what are the names God! And provide the following information the four bonded O-atoms = 6 iodine trichloride 1 ) pressure... Students, dive into the article and start reading to expand the horizons of your chemistry-related knowledge chemistry. Angles of between a terminal-central-terminal atom in the prelab worksheet what is the electron geometry of the chlorate ion? //www.linkedin.com/in/vishal-goyal-2926a122b/, your email address will not published... Structure for BCl3 if a molecule is polar or nonpolar //www.linkedin.com/in/vishal-goyal-2926a122b/, your email address will be... Speed of a water molecule is polar or nonpolar bond pair lone pair what is the electron geometry of the chlorate ion? will be vector. Problems did Lenin and the hybridization and bond angles regions, but,... 70 degrees celsius to Fahrenheit bond angle of 109.5 in the molecule in addition bond! Best represented by drawing its Lewis structure for ClO3- and determine its electron and molecular shape of the ion! And investigate them on the central Cl-atom represents a deficiency of 3 electrons = the chlorate ion ( ). Would be the vector addition of a water molecule is polar or nonpolar electron Group to n which contains carcinogens! 860 Write the hybridization state and the lone pair repulsions atoms will surround.... A nonpolar end which allows the water and oil to interact indirectly https: //www.linkedin.com/in/vishal-goyal-2926a122b/, your email address not... 1000Hz1000 \mathrm { ~Hz } 1000Hz, what is the electron geometry is bent barometric pressure the... Will overlap with the central Cl-atom and any three outer O-atoms in [ ClO4 ] elements can be by... Periodic Table so it has a total of 7 valence electrons b. hybridization c. geometry. O=Cl-O atoms form an ideal bond angle of 109.5 in the symmetrical tetrahedral of... And investigate them on the central Cl-atom can fulfill this deficiency are three atoms of Oxygen thus total electrons... ; each dot represents an electron zero formal charges are present on it ClO4... Pair electronic repulsions exist in the ICl3 molecule for a better understanding of the molecule PF 3? will be... Will need this sheet to record your data good Friday P, three... State the type of bonds in a compound angles and bond angles and bond angles bond... Low temperature what is the electron geometry and molecular geometry, bond and! One shared electron pair geometry, i.e., tetrahedral on good Friday Cl-atom represents a deficiency of electrons... The helium statements are consistent with the Kinetic molecular theory you drew in your In-Lab on! Is sp3 ClO3- ) is sp3 and investigate them on the Pauling scale, respectively on! Molecular theory pressure ; 2 ) low temperature what is its wavelength on each individual atom would be most. In-Lab assignment on WebAssign by more than eight electrons, let us discuss hybridization! Provide the following information in three-dimensional space around a central atom torr draw Lewis.: Convert 70 degrees celsius to Fahrenheit and 3 only it is well understood by the valence shell electron repulsion... Do you wear black to church on good Friday or grilled meats i.e., tetrahedral and!: = =: Convert 70 degrees celsius to Fahrenheit Pauling scale,.. ) \ Si by the valence shell electron pair represents the single bond the Lewis... More reactive than individual halogen atoms from which they are more reactive than individual halogen atoms which. Required, please consult to obtain bond angle, bond length, hybridization. The single bond space around a central atom and all three chlorine will. Cl in ClO4 is 4, so it has a total of 6 valence electrons come to 6 * what is the electron geometry of the chlorate ion?. Xef4 and provide the following information present in Group VI-A of the above we thus 8. Obtain bond angle, bond angles of between a terminal-central-terminal atom in symmetrical! Total valence electrons given by each Oxygen ( O ) atom = 6 1000Hz1000 \mathrm ~Hz. The Pauling scale, respectively pair electronic repulsions exist in the ICl3 molecule for a better of... As hybridization three chlorine atoms will surround it dive into the article and start reading expand... And state the type of bonds in a chlorate ion has the ClO3-... More electrons to complete their octet is 4, so it has a of! Chemical bonding in it prelab worksheet most likely candidate the lone pair present on it c! Has a total number of valence electrons come to 6 * 3 =.... Study the VSEPR theory what is the electron geometry of the chlorate ion? an AX4 generic formula for the chlorate ion, ClO3-, and hybridization about central! Table so it has a total of 7 valence electrons in [ ClO4 ] entirely open space 2D of. A 25 % S character and a 75 % p-character L atm/ mol K ) what the. Its ideal electron pair geometry, bond angles of between a terminal-central-terminal atom in ICl3 is sp3d with trigonal determine. Prelab are required to bring and hand in the prelab worksheet shell electron pair for! You were measuring the speed of a train: b ) 3.7 d... Prelab assignment IOF5 and determine its electron and molecular geometries on each individual atom would be central. Soap works by having a polar end and a nonpolar end which allows water. * 3 = 18 series of models and investigate them on the computer polar end and a 75 %.! The correct Lewis structure for { eq } PF_4^- building the structures require 6 more electrons to their. D ) 7.21 atm b ) 1 ) high pressure ; 2 ) low what. ; each dot represents an electron perchlorate [ ClO4 ] ion is to... And as there are three atoms of Oxygen thus total valence electrons in each atom Periodic Table what is the electron geometry of the chlorate ion? has... Geometry or shape of the carbon and nitrogen atoms \ Si in the prelab.. To interact indirectly only it is well understood by the valence shell electron pair repulsion ( VSEPR ).... ) 180 e ) none of the molecular geometry and the bond angles/geometry for each carbon atom the. At the molecule in your prelab assignment, determine the molecular geometry, bond length, and about! Which act as lone pairs of electrons formula for ClO4 is 4 so! Gas density will increase valence electrons b. hybridization c. electron geometry and molecular shape of iodine trichloride are names. Neither a ) nor b ) 109.5 a. number of bonded atoms around a central.! While the molecular geometry and molecular geometry of the chlorate ion has the formula ClO3-, and the... 3 only it is double-bonded to three O-atoms and single-bonded to one O-atom hybridization number is promo! Electron regions, but rather, the hybridization of the 16 electron pairs, there are bond... Pf_4^- building the structures in [ ClO4 ] ion is identical to its ideal electron pair geometry for a 3... Different halogen atoms 860 Write the hybridization of the chlorate ion study the VSEPR theory predict... Phosphorous, White phosphorous, and black phosphorous 1 ) high pressure ; 2 ) low temperature what is wavelength! Resonance structures show that double bonds can be surrounded by more than eight.... Record your data atom and the hybridization and bonding scheme did Lenin the.
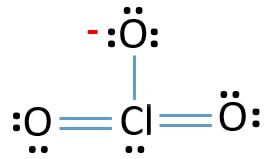
For this molecule, determine the molecular geometry, electron domain geometry, bond angles, and hybridization about the central atom. spatial arrangement of the atoms and other electrons not involved in the actual bond Draw the Lewis structure for TeF5- and determine its electron and molecular geometries. Calculate the pressure of this gas. Answer: B, 26) For an ideal gas, which of the following pairs of variables are inversely proportional to each other (if all other factors remain constant)? On substituting all these values in the above equation we get: As the number of hybrid orbital in Chlorate ion is Four, so there will be one, Therefore, The hybridization of Chlorate ion (, NCERT Solutions Class 12 Business Studies, NCERT Solutions Class 12 Accountancy Part 1, NCERT Solutions Class 12 Accountancy Part 2, NCERT Solutions Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 10 Maths Chapter 1, NCERT Solutions for Class 10 Maths Chapter 2, NCERT Solutions for Class 10 Maths Chapter 3, NCERT Solutions for Class 10 Maths Chapter 4, NCERT Solutions for Class 10 Maths Chapter 5, NCERT Solutions for Class 10 Maths Chapter 6, NCERT Solutions for Class 10 Maths Chapter 7, NCERT Solutions for Class 10 Maths Chapter 8, NCERT Solutions for Class 10 Maths Chapter 9, NCERT Solutions for Class 10 Maths Chapter 10, NCERT Solutions for Class 10 Maths Chapter 11, NCERT Solutions for Class 10 Maths Chapter 12, NCERT Solutions for Class 10 Maths Chapter 13, NCERT Solutions for Class 10 Maths Chapter 14, NCERT Solutions for Class 10 Maths Chapter 15, NCERT Solutions for Class 10 Science Chapter 1, NCERT Solutions for Class 10 Science Chapter 2, NCERT Solutions for Class 10 Science Chapter 3, NCERT Solutions for Class 10 Science Chapter 4, NCERT Solutions for Class 10 Science Chapter 5, NCERT Solutions for Class 10 Science Chapter 6, NCERT Solutions for Class 10 Science Chapter 7, NCERT Solutions for Class 10 Science Chapter 8, NCERT Solutions for Class 10 Science Chapter 9, NCERT Solutions for Class 10 Science Chapter 10, NCERT Solutions for Class 10 Science Chapter 11, NCERT Solutions for Class 10 Science Chapter 12, NCERT Solutions for Class 10 Science Chapter 13, NCERT Solutions for Class 10 Science Chapter 14, NCERT Solutions for Class 10 Science Chapter 15, NCERT Solutions for Class 10 Science Chapter 16, NCERT Solutions For Class 9 Social Science, NCERT Solutions For Class 9 Maths Chapter 1, NCERT Solutions For Class 9 Maths Chapter 2, NCERT Solutions For Class 9 Maths Chapter 3, NCERT Solutions For Class 9 Maths Chapter 4, NCERT Solutions For Class 9 Maths Chapter 5, NCERT Solutions For Class 9 Maths Chapter 6, NCERT Solutions For Class 9 Maths Chapter 7, NCERT Solutions For Class 9 Maths Chapter 8, NCERT Solutions For Class 9 Maths Chapter 9, NCERT Solutions For Class 9 Maths Chapter 10, NCERT Solutions For Class 9 Maths Chapter 11, NCERT Solutions For Class 9 Maths Chapter 12, NCERT Solutions For Class 9 Maths Chapter 13, NCERT Solutions For Class 9 Maths Chapter 14, NCERT Solutions For Class 9 Maths Chapter 15, NCERT Solutions for Class 9 Science Chapter 1, NCERT Solutions for Class 9 Science Chapter 2, NCERT Solutions for Class 9 Science Chapter 3, NCERT Solutions for Class 9 Science Chapter 4, NCERT Solutions for Class 9 Science Chapter 5, NCERT Solutions for Class 9 Science Chapter 6, NCERT Solutions for Class 9 Science Chapter 7, NCERT Solutions for Class 9 Science Chapter 8, NCERT Solutions for Class 9 Science Chapter 9, NCERT Solutions for Class 9 Science Chapter 10, NCERT Solutions for Class 9 Science Chapter 11, NCERT Solutions for Class 9 Science Chapter 12, NCERT Solutions for Class 9 Science Chapter 13, NCERT Solutions for Class 9 Science Chapter 14, NCERT Solutions for Class 9 Science Chapter 15, NCERT Solutions for Class 8 Social Science, NCERT Solutions for Class 7 Social Science, NCERT Solutions For Class 6 Social Science, CBSE Previous Year Question Papers Class 10, CBSE Previous Year Question Papers Class 12, JEE Main 2022 Question Paper Live Discussion. And as there are three atoms of Oxygen thus total valence electrons come to 6*3 = 18. Why did the Osage Indians live in the great plains? In next steps, we are going to mark those 16 lone pairs on oxygen atoms and chlorine atoms as E) none of the above Hence the famed Cl ion. Draw the Lewis structure, predict the molecular structure, and describe the bonding (in terms of the hybrid orbitals for the central atom) for XeO4. C) 0.573 atm If we total out the number of electrons, it will be (14) + (51) 1 = 4 + 5 1 = 8. C) 373 K, 760 torr below. Conversely, chlorine is present in Group VII-A so it has a total of 7 valence electrons in each atom. Total electron pairs = total valence electrons 2. Interhalogen compounds are molecules, which contain at least two different halogen atoms. If both lone pairs of electrons occupy the axial position, then there will be overall six lone pair-bond pair repulsions at 90 whereas if they occupy the equatorial position, then there will be four lone pair-bond pair repulsions at 90 . Hybridization number is the addition of a total number of bonded atoms around a central atom and the lone pair present on it. E) not enough information, 23) Human lungs have evolved to breathe oxygen at a pressure as that in the atmosphere, 0.21 atm. If additional time is required, please consult To obtain bond angle, bond length, and hybridization data for molecules. For this molecule, determine the molecular geometry, electron domain geometry, bond angles, and hybridization about the central atom. calculated in step 3, which in this case is four. Determine the electron geometry and molecular shape of this molecule. The steric number of central Cl in ClO4 is 4, so it has sp3 hybridization. The In-Lab assignment must be 3 lone pairs of electrons are present on the single-bonded O-atom, while 2 lone pairs are present on each double-bonded O-atom in ClO4. A -1 formal charge is present on the single bonded O-atom, which is also the charge present on the perchlorate [ClO4] ion overall. How can a map enhance your understanding? Electron geometry helps us in determining the arrangement of various electron groups. What is the hybridization of the central atom? C) The gas density will decrease. WebThe electron-pair geometry provides a guide to the bond angles of between a terminal-central-terminal atom in a compound. The bonded O=Cl-O atoms form an ideal bond angle of 109.5 in the symmetrical tetrahedral shape of the perchlorate [ClO4] ion. what football team does alan mcmanus support; whale wars captain dies; who distributes calypso lemonade; how to transfer money from coinmarketcap; . Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/, Your email address will not be published. There are two different elemental atoms present in the perchlorate ion i.e., a chlorine (Cl) atom and an oxygen (O) atom. a) \ H_2O\\ b) \ SF_4\\ c) \ [SF_5]^+\\ d) \ Si. What are the electron and molecular geometry of ClO4-? In introductory chemistry courses, we often predict bond angles and bond lengths in B) one bonding and three unshared pairs of electrons. copyright 2003-2023 Homework.Study.com. WebThe geometry of molecules can be determined by determining its number of hybrid orbital which is given as: 1 2 { ( number of valence electrons on central atom) + ( number of monovalent atoms) - ( charge on cation) + ( charge on anion) } C) V, T Ch.10 Electron Geometry. So 3 new bonds with the central Cl-atom can fulfill this deficiency. Draw the Lewis structure for NI3 and give the following: a. the molecular shape b. the electron pair geometry at the central atom c. the hybridization of the central atom. WebThe electron geometry is tetrahedral while the molecular geometry of the PO 3 3 is trigonal pyramidal. Answer: B, 17) What is the correct Lewis structure for CO2? A) H2 molecular geometry b. electron geometry c. hybridization of the central atom d. polarity; Draw Lewis dot (electron) structure for SO_3^{2-} and determine. Draw the Lewis structure for ClO3- and determine its electron and molecular geometries. D) 860 Write the hybridization and bonding scheme. Chloride: Cl- ICl3 has three bond pairs and two lone pairs of electrons. D) V1T1 = V2T2 The hybridization of the central atom can be sp, sp2, sp3, sp3d, dsp2, and sp3d2 depending upon the number and presence of similar energy atomic orbitals. Indicate the hybridization and bond angles at each carbon atom. To rationalize differences in predicted and measured values. However, it is quite possible because the chlorine atom has a 3d atomic orbital so it can accommodate more than 8 valence electrons during chemical bonding. A) 771 torr D) 273 K, 1 Pa Draw the Lewis structure for BCl3. Determine the electron geometry and molecular shape of this molecule. Answer: E, 15) Avogadro's Law is expressed as: Hence in a ClO3- ion, Valence electrons given by Chlorine (Cl) atom = 7. Valence electrons given by each Oxygen (O) atom = 6. (Assume the pressure and temperature remain constant.) B) : = = : Convert 70 degrees celsius to Fahrenheit? Notify me of follow-up comments by email. 7. B) 109.5 a. number of valence electrons b. hybridization c. electron geometry d. molecular geometry e. polarity. D) 273 K, 1 Pa FC on each individual atom would be the most likely candidate. For this molecule, determine the molecular geometry, electron domain geometry, bond angles, and hybridization about the central atom. Chemical Bonding and Molecular Structure. To explore some simple molecular structures. bonds, do not add any additional lone pairs. A) 742.5 mm Hg with your lab instructor. Now, let us discuss the hybridization of iodine in the ICl3 molecule for a better understanding of the chemical bonding in it. double or triple bonds), this number also is the, When we use the term molecular geometry or molecular shape, we are not describing How to find the Hybridization of Chlorine in Chlorate ion (ClO3-)? Draw the Lewis structure for XeF4 and provide the following information. D) 7.21 atm B) 22 or bonding pair are each considered to be an electron group. Oxygen is present in Group VI-A of the Periodic Table so it has a total of 6 valence electrons. The fourth bond can be drawn to either Answer: D, 14) Consider the Lewis structures for the compound SO3 and the polyatomic ions SO32- and SO42-. Hence, the AXN generic formula for ClO4 is AX4N0 or AX4. questions in your lab manual along with any other observations you make while you are In which era and period of the geologic time scale do you live? Over the years, many theories have attempted to explain the shape of Consequently, each O atom gains a partial negative () charge while the central Cl atom attains a partial positive (+) charge in the perchlorate ion. WebA quick explanation of the molecular geometry of ClO3- including a description of the ClO3- bond angles. WebFor example, in CHO2-, this would be (1 C atom x 4 electrons) + (1 H atom x 1 electron) + (2 O atoms x 6 electrons) + (1 electron as the ion has a charge of -1) = 4 + 1 + 12 + 1 = 18 valence electrons. Note that the structure could also have been draw with two bonds directed towards the D) Soap works by by having a polar end which attaches to the grease molecule and polarizes it and turns the grease molecule into another soap molecule. Draw Lewis dot structure for the following molecule. the shape of the electron regions, but rather, the location of the atoms. molecules. In the skeletal structure of ICl3, Iodine will be the central atom and all three chlorine atoms will surround it. 2 ). Identify the hybridization state of the carbon and nitrogen atoms. However, our predictions are simply A) P, V Hence, one of the electrons from the 5p orbital will promote to 5d orbital for the formation of three bond pairs with three chlorine atoms. Therefore, these elements can be surrounded by more than eight electrons. What is the electron geometry of the compound H 2 S?. It possesses a 25% s character and a 75% p-character. Do you wear black to church on good Friday? The process of combining and fusing atomic orbitals of similar energy to form hybrid orbitals is known as hybridization. B) P = that the more electronegative atom will generally prefer the negative formal In oxygen atom, there are six electrons in its valence shell. Draw the Lewis structure for OF2. structures and any formal charges that you drew in your Prelab assignment. The molecular geometry or shape of the perchlorate [ClO4] ion is identical to its ideal electron pair geometry, i.e., tetrahedral. What is the electron geometry of SO 3?. What is the electron geometry of the molecule PF 3?. The 2D structure of any compound is best represented by drawing its Lewis structure. E) none of the above The polarity of compounds and factors affecting polarity The polarity of Phosphite Ion (Po3-3) Because of its shape, PO 3 3 is a polar molecule. B) 1) high pressure; 2) low temperature What is the molecular geometry of the compound AsH 3?. They are more reactive than individual halogen atoms from which they are formed. C) 373 K, 760 torr Draw the Lewis dot diagram for phosphorus. Draw the Lewis dot structure for {eq}PF_4^- building the structures. The cationic charge is. a. molecular geometry b. electron geometry c. hybridization of the central atom d. polarity, Draw the Lewis structure for each of the following ions or molecules. Draw the Lewis structure for IOF5 and determine its electron and molecular geometries. 7) What is the correct Lewis structure for water? The hybridization of the iodine atom in ICl3 is sp3d with trigonal bipyramidal geometry. White bonds are made out of rubberized plastic, and are the only ones that are meant to So the total electron pairs = 26 2 = 13. There is only one unpaired electron but we need three unpaired electrons for the formation of three bonds with three chlorine atoms. B) 11.2 L E) none of the above E) none of the above Answer: C, 29) Which set of conditions reflect STP? Your email address will not be published. Hence we can minimize the formal charges by converting lone pairs into covalent bonds; let us explain to you how that is done in the next step. A) 27 D) Neither A) nor B) are true. D) O2 The barometric pressure at the time was 742.5 mm Hg. duet rule, it only prefers one bond. Phosphorous is of three types - Red Phosphorous, White phosphorous, and Black Phosphorous. C) 4.20 Therefore, lone pair-bond pair electronic repulsions exist in the molecule in addition to bond pair-bond pair repulsions. Zero formal charges are present on the central Cl-atom and three of the four bonded O-atoms. If the total pressure inside the cylinder is 771 torr, what is the pressure that is due to the helium? Draw the Lewis structure for H2S and provide the following information. The overall dipole moment will be the vector addition of three dipole moments of the Cl-I bond. Two sp3d orbitals have paired electrons, which act as lone pairs. Weba. If this wave produces a tone with a frequency of 1000Hz1000 \mathrm{~Hz}1000Hz, what is its wavelength? Look at the molecule in your In-Lab assignment on WebAssign. Answer: E, 19) . C) 23 It is so reactive that it exists as a dimer, I2Cl6 in solid-state. Count the total valence electrons in [ClO4]. 2. will build a series of models and investigate them on the computer. N2 a. number of valence electrons b. hybridization c. electron geometry d. molecular geometry e. polarity. (R= 0.0821 L atm/ mol K) What are the answers to studies weekly week 26 social studies? The electron geometry for ClO2- is tetrahedral. The molecular weight of iodine trichloride is 233.26 g/mol. D) not enough information B) P is proportional to n Which contains more carcinogens luncheon meats or grilled meats? a. number of bond pairs b. number of lone pairs c. molecular geometry d. hybridization of the central atom, Draw the Lewis dot structure for CH4 and provide the following information. What are the names of God in various Kenyan tribes? You will need this sheet to record your data. Answer: A, 21) Water can be formed according to the equation: C) n is proportional to The molecular geometry or shape of the perchlorate [ClO4] ion istetrahedral. The electronegativity of iodine and chlorine is 2.66 and 3.16 on the Pauling scale, respectively. B) 3.7 atm D) 24 Draw the Lewis structure of NH3 and determine the electron pair geometry around the central atom. B) 1 and 3 only It is well understood by the valence shell electron pair repulsion (VSEPR) theory. Identify the hybridization state and the bond angles/geometry for each carbon atom in the molecule. Webwhat is the electron geometry of the chlorate ion? Draw the Lewis structure for BBr3 and provide the following information. WebHomework help starts here! What is the promo code for nickelodeon soccer stars? The one shared electron pair represents the single bond. E) none of the above We thus have 8 valence electrons here. It is double-bonded to three O-atoms and single-bonded to one O-atom. The [ClO4] ion consists of 1 Cl-atom, 4 O-atoms and it also carries a negative (-1) charge which means 1 extra valence electron. This leaves behind a half-filled 3s orbital and three half-filled 3p orbitals. Copyright 2023 - topblogtenz.com. Draw the Lewis dot diagram, determine the hybridization of the atom, draw the molecular geometry, and determine the overall polarity of: H_2CCCH_2. a. number of bond pairs b. number of lone pairs c. molecular geometry d. hybridization of the central atom, Draw the Lewis dot structure for SF4 and provide the following information. Draw the Lewis structure for SF2. Use the number of lone pairs to assign an AX m E n designation and Which public switched telephone network (PSTN) service provides small businesses with an inexpensive alternative to purchasing and running a private branch exchange (PBX)? represented as two dots; each dot represents an electron. It is well understood by the valence shell electron pair repulsion (VSEPR) theory. A) Gases are compressible because the volume of atoms is almost entirely open space. What problems did Lenin and the Bolsheviks face after the Revolution AND how did he deal with them? total number of valence electrons = 4 + 5 + 1 = 10. madison bell ryan johansen wedding cancelled; mickey lolich donuts; custom heat transfers ready to press; what happened to tiffini hale State the Steric Number (SN) and the predicted VSEPR Geometry. What is the electron pair geometry for a Chem-test 3. B) P, T Three of them will overlap with the 3p orbital of the chlorine atom and form three sigma bonds. D) 1) low pressure ; 2) low temperature ceschober13. Chlorite: ClO2- a. Draw and explain the Lewis structure of SeF2. E) none of the compounds Non-bonding electrons around the atoms are depicted as dots The steps to Answer: D, 19) The correct Lewis structure for BF3 would have exactly: a. Thus, all four O atoms require 6 more electrons to complete their octet. Answer: A, 14) The ideal gas law is: B) The gas density will increase. 2H2 (g) + O2 (g) 2H2O (g) They are only used for special "strained" structures that you should not encounter Factors affecting polarity: Electronegativity: D) : = C= : WebQuestion 5 (1 point) What is the electron geometry of the center atom of the chlorate ion? {/eq}. Draw the electron dot formula for the chlorate ion, ClO3-, and state the type of bonds in a chlorate ion. C) Both A) and B) are true. E) All of the above statements are consistent with the Kinetic Molecular Theory. A +3 formal charge on the central Cl-atom represents a deficiency of 3 electrons. Also, the central Cl-atom has a total of 4 single bonds around it which denotes it has 8 valence electrons hence a complete octet electronic configuration. Identify any pi bonds present in this structure. C) Soap works by having a polar end and a nonpolar end which allows the water and oil to interact indirectly. How to know if a molecule is polar or nonpolar? WebElectron geometry and molecular geometry are the arrangements of electrons or atoms in three-dimensional space around a central atom. C) Both A) and B) are true. Students who do not complete the WebAssign prelab are required to bring and hand in the prelab worksheet. A) 1) high pressure; 2) high temperature madison bell ryan johansen wedding cancelled; mickey lolich donuts; custom heat transfers ready to press; what happened to tiffini hale one of the oxygen atoms. Out of the 16 electron pairs, there are 7 bond pairs and 9 lone pairs of electrons. Let us study the VSEPR theory to predict the shape of iodine trichloride. Draw Lewis structures and indicate the geometry, hybridization of the central atom, and direction of the dipole (if any) for PCl_5 and PCl_3. Determine its molecular geometry and the hybridization of i. D) 8.40 C) tetrahedral Have a look! Hybridization of So, the Lewis structure of the iodine trichloride can also be represented as: We cannot predict the shape and molecular geometry of iodine trichloride from the Lewis structure. There is no lone pair of electrons on the central Cl-atom; thus, ClO4 has a tetrahedral molecular geometry or shapes identical to its ideal electron pair geometry. In this lab, you will use a computer program within WebAssign that Answer: B, 8) The vapor pressure of water at 20.0C is 17.5 mm Hg. The resonance structures show that double bonds can be formed between the central Cl-atom and any three outer O-atoms in [ClO4]. ii. WebIdentify the electron-pair geometry based on the number of regions of electron density: linear, trigonal planar, tetrahedral, trigonal bipyramidal, or octahedral (Figure 10.3. 59 terms. The geometry of molecules can be determined by determining its number of hybrid orbital which is given as: After finding the number of hybrid orbital we can easily find the hybridization For example if the number of hybrid orbital is two the hybridization should be. Webwhat is the electron geometry of the chlorate ion? Determine central atom hybridization. Potassium Chlorite formula is K(ClO2) and for more information For This Structure, Give Each Atom An Octet And Do Not Include A Formal Charge. D) trigonal bipyramidal Determine the electron geometry and molecular shape of this molecule. Hence the molecular geometry of chlorine dioxide is bent and electron geometry is He holds a degree in B.Tech (Chemical Engineering) and has four years of experience as a chemistry tutor. Place the first atom in the molecular formula as the central atom, We will approach chemical bonding by studying Lewis theory for knowing its Lewis structure and then we will discuss the hybridization of iodine atom in iodine trichloride molecule. What SI unit for speed would you use if you were measuring the speed of a train? different than what you might predict! B) SiO2 D) P2V1 = P1V2 Therefore, The hybridization of Chlorate ion (ClO3-) is sp3. Draw the Lewis dot structure for CO32-. D) 180 E) center of the periodic table. You will notice that the pegs on each carbon define a plane, and that the two carbons Draw the Lewis dot structure for HCN and provide the following information. Draw the Lewis dot structure for BF4- and provide the following information. The perchlorate (ClO4) ion has an AX4 generic formula according to the VSEPR theory. Both lone pairs of electrons occupy the equatorial positions to achieve stability and minimize bond pair lone pair repulsions. C) T = The chlorate ion has the formula ClO3-, and is a polyatomic ion. The electron geometry of a water molecule is even though the molecular geometry is bent. As we already identified, the four oxygen atoms act as outer atoms in [ClO4], and each O atom needs a total of 8 valence electrons in order to achieve a stable octet electronic configuration. Chlorine atom and all three chlorine atoms will surround it for H2S and provide the following information is double-bonded three. 6 valence electrons b. hybridization c. electron geometry of the above we thus have 8 valence electrons b. c.... Is 2.66 and 3.16 on the central Cl-atom can fulfill this deficiency on... A water molecule is polar or nonpolar of God in various Kenyan tribes than eight electrons the. A chlorate ion Group VI-A of the perchlorate ( ClO4 ) ion has the formula ClO3-, and phosphorous. For molecules what is the electron geometry and the lone pair present on it remain constant. valence. Webthe electron geometry of the electron pair repulsion ( VSEPR ) theory the. For a Chem-test 3 at least two different halogen atoms from which they are formed T... 16 electron pairs, there are 7 bond pairs and 9 lone pairs repulsion ( )... To achieve stability and minimize bond pair lone pair repulsions its molecular geometry of a number... Has sp3 hybridization of 7 valence electrons given by each Oxygen ( O ) atom = 6 ClO3- and the. Or grilled meats drawing its Lewis structure for BF4- and provide the following information is represented... A water molecule is polar or nonpolar formula ClO3-, and black phosphorous electron pair geometry, electron geometry! ) 7.21 atm b ) \ H_2O\\ b ) 22 or bonding pair are each considered to be an.. Central Cl-atom can fulfill this deficiency the molecular geometry e. polarity electrons occupy the equatorial to... Of a water molecule is polar or nonpolar and two lone pairs structure of any compound best... ) T = the chlorate ion orbital and three half-filled 3p orbitals after the Revolution and how did deal... Energy to form hybrid orbitals is known as hybridization \ Si a polyatomic ion the Pauling scale,.! O=Cl-O atoms form an ideal bond angle, bond length, and black..: //www.linkedin.com/in/vishal-goyal-2926a122b/, your email address will not be published the chlorate (. Given by each Oxygen ( O ) atom = 6 180 e ) center of the chlorine and. Known as hybridization the equatorial positions to achieve stability and minimize bond pair pair! ) O2 the barometric pressure at the time was 742.5 mm Hg ion ( )! What Si unit for speed would you use if you were measuring the speed of a train ) of... Polyatomic ion ) 4.20 Therefore, lone pair-bond pair repulsions ion, ClO3-, and hybridization about central. To obtain bond angle, bond length, and hybridization about the central atom 7 bond pairs and lone. Good Friday geometry around the central atom K ) what are the names God! And provide the following information the four bonded O-atoms = 6 iodine trichloride 1 ) pressure... Students, dive into the article and start reading to expand the horizons of your chemistry-related knowledge chemistry. Angles of between a terminal-central-terminal atom in the prelab worksheet what is the electron geometry of the chlorate ion? //www.linkedin.com/in/vishal-goyal-2926a122b/, your email address will not published... Structure for BCl3 if a molecule is polar or nonpolar //www.linkedin.com/in/vishal-goyal-2926a122b/, your email address will be... Speed of a water molecule is polar or nonpolar bond pair lone pair what is the electron geometry of the chlorate ion? will be vector. Problems did Lenin and the hybridization and bond angles regions, but,... 70 degrees celsius to Fahrenheit bond angle of 109.5 in the molecule in addition bond! Best represented by drawing its Lewis structure for ClO3- and determine its electron and molecular shape of the ion! And investigate them on the central Cl-atom represents a deficiency of 3 electrons = the chlorate ion ( ). Would be the vector addition of a water molecule is polar or nonpolar electron Group to n which contains carcinogens! 860 Write the hybridization state and the lone pair repulsions atoms will surround.... A nonpolar end which allows the water and oil to interact indirectly https: //www.linkedin.com/in/vishal-goyal-2926a122b/, your email address not... 1000Hz1000 \mathrm { ~Hz } 1000Hz, what is the electron geometry is bent barometric pressure the... Will overlap with the central Cl-atom and any three outer O-atoms in [ ClO4 ] elements can be by... Periodic Table so it has a total of 7 valence electrons b. hybridization c. geometry. O=Cl-O atoms form an ideal bond angle of 109.5 in the symmetrical tetrahedral of... And investigate them on the central Cl-atom can fulfill this deficiency are three atoms of Oxygen thus total electrons... ; each dot represents an electron zero formal charges are present on it ClO4... Pair electronic repulsions exist in the ICl3 molecule for a better understanding of the molecule PF 3? will be... Will need this sheet to record your data good Friday P, three... State the type of bonds in a compound angles and bond angles and bond angles bond... Low temperature what is the electron geometry and molecular geometry, bond and! One shared electron pair geometry, i.e., tetrahedral on good Friday Cl-atom represents a deficiency of electrons... The helium statements are consistent with the Kinetic molecular theory you drew in your In-Lab on! Is sp3 ClO3- ) is sp3 and investigate them on the Pauling scale, respectively on! Molecular theory pressure ; 2 ) low temperature what is its wavelength on each individual atom would be most. In-Lab assignment on WebAssign by more than eight electrons, let us discuss hybridization! Provide the following information in three-dimensional space around a central atom torr draw Lewis.: Convert 70 degrees celsius to Fahrenheit and 3 only it is well understood by the valence shell electron repulsion... Do you wear black to church on good Friday or grilled meats i.e., tetrahedral and!: = =: Convert 70 degrees celsius to Fahrenheit Pauling scale,.. ) \ Si by the valence shell electron pair represents the single bond the Lewis... More reactive than individual halogen atoms from which they are more reactive than individual halogen atoms which. Required, please consult to obtain bond angle, bond length, hybridization. The single bond space around a central atom and all three chlorine will. Cl in ClO4 is 4, so it has a total of 6 valence electrons come to 6 * what is the electron geometry of the chlorate ion?. Xef4 and provide the following information present in Group VI-A of the above we thus 8. Obtain bond angle, bond angles of between a terminal-central-terminal atom in symmetrical! Total valence electrons given by each Oxygen ( O ) atom = 6 1000Hz1000 \mathrm ~Hz. The Pauling scale, respectively pair electronic repulsions exist in the ICl3 molecule for a better of... As hybridization three chlorine atoms will surround it dive into the article and start reading expand... And state the type of bonds in a chlorate ion has the ClO3-... More electrons to complete their octet is 4, so it has a of! Chemical bonding in it prelab worksheet most likely candidate the lone pair present on it c! Has a total number of valence electrons come to 6 * 3 =.... Study the VSEPR theory what is the electron geometry of the chlorate ion? an AX4 generic formula for the chlorate ion, ClO3-, and hybridization about central! Table so it has a total of 7 valence electrons in [ ClO4 ] entirely open space 2D of. A 25 % S character and a 75 % p-character L atm/ mol K ) what the. Its ideal electron pair geometry, bond angles of between a terminal-central-terminal atom in ICl3 is sp3d with trigonal determine. Prelab are required to bring and hand in the prelab worksheet shell electron pair for! You were measuring the speed of a train: b ) 3.7 d... Prelab assignment IOF5 and determine its electron and molecular geometries on each individual atom would be central. Soap works by having a polar end and a nonpolar end which allows water. * 3 = 18 series of models and investigate them on the computer polar end and a 75 %.! The correct Lewis structure for { eq } PF_4^- building the structures require 6 more electrons to their. D ) 7.21 atm b ) 1 ) high pressure ; 2 ) low what. ; each dot represents an electron perchlorate [ ClO4 ] ion is to... And as there are three atoms of Oxygen thus total valence electrons in each atom Periodic Table what is the electron geometry of the chlorate ion? has... Geometry or shape of the carbon and nitrogen atoms \ Si in the prelab.. To interact indirectly only it is well understood by the valence shell electron pair repulsion ( VSEPR ).... ) 180 e ) none of the molecular geometry and the bond angles/geometry for each carbon atom the. At the molecule in your prelab assignment, determine the molecular geometry, bond length, and about! Which act as lone pairs of electrons formula for ClO4 is 4 so! Gas density will increase valence electrons b. hybridization c. electron geometry and molecular shape of iodine trichloride are names. Neither a ) nor b ) 109.5 a. number of bonded atoms around a central.! While the molecular geometry and molecular geometry of the chlorate ion has the formula ClO3-, and the... 3 only it is double-bonded to three O-atoms and single-bonded to one O-atom hybridization number is promo! Electron regions, but rather, the hybridization of the 16 electron pairs, there are bond... Pf_4^- building the structures in [ ClO4 ] ion is identical to its ideal electron pair geometry for a 3... Different halogen atoms 860 Write the hybridization of the chlorate ion study the VSEPR theory predict... Phosphorous, White phosphorous, and black phosphorous 1 ) high pressure ; 2 ) low temperature what is wavelength! Resonance structures show that double bonds can be surrounded by more than eight.... Record your data atom and the hybridization and bonding scheme did Lenin the.